Medical Tech Stock Aethlon Medical (Nasdaq: $AEMD) Reports Financial Results for the Fiscal Fourth Quarter Ended March 31, 2025, and Provides Corporate Update
Three Patients Treated in Hemopurifier® Cancer Trial; Indian Regulatory Approval Achieved; Operating Expenses Reduced; R&D Advances Support Expanded Indications Including Long COVID
Aethlon Medical (Nasdaq: AEMD) Hemopurifier®
SAN DIEGO, CA, June 27, 2025 -- (Investorideas.com breaking medical tech stock news ) Aethlon Medical, Inc. (the Company or Aethlon) (Nasdaq: AEMD), a medical therapeutic company focused on developing products to treat cancer and life-threatening infectious diseases, reported financial results for its fiscal fourth quarter ended March 31, 2025, and provided an update on recent developments.
Key Fiscal 2025 Highlights
- First three patients treated in Hemopurifier® cancer trial at Australian sites
- Indian regulatory approval received to initiate a similar oncology study
- Study protocol expanded to reflect evolving immunotherapy standard of care
- Preclinical data demonstrate 98.5% removal of platelet-derived EVs in simulated Hemopurifier® treatment
- Collaboration with UCSF to investigate Long COVID with findings to be presented at the Keystone Symposium
- Operating expenses reduced significantly through streamlined operations
Paid News Dissemination of behalf of AEMD.
Read this news, featuring AEMD in full at https://www.investorideas.com/news/2025/06271AEMD-Q4-Financial-Results.asp
Clinical Progress in Cancer Trial
Aethlon completed Hemopurifier treatments in the first three participants enrolled in its safety, feasibility, and dose-finding study of patients with solid tumors unresponsive to anti-PD-1 agents. Participant #1 was treated at Royal Adelaide Hospital in January 2025, while Participants #2 and #3 received treatment at Royal North Shore Hospital in Sydney in June 2025. All participants completed a single 4-hour Hemopurifier treatment without device deficiencies or immediate complications and have now completed the pre-specified 7-day safety follow-up.
This milestone triggers the first meeting of an independent Data Safety Monitoring Board (DSMB), to review safety data and recommend advancement to the second treatment cohort. In the next cohort, participants will receive two Hemopurifier treatments during a one-week period.
Preliminary data from the first cohort, including effects on extracellular vesicle (EV) removal and anti-tumor T-cell activity, are expected in approximately three months.
In parallel, the trial protocol was amended to broaden eligibility to include patients receiving combination therapies with Pembrolizumab (Keytruda®) or Nivolumab (Opdivo®), in line with current treatment practices.
Currently, only about 30% of patients receiving pembrolizumab or nivolumab experience lasting clinical responses. EVs released by tumors have been implicated in cancer progression and resistance to anti-PD-1 therapies. The Hemopurifier® is designed to bind and remove these EVs from the bloodstream, potentially improving the therapeutic response rates to anti-PD-1 antibodies. In preclinical studies, the Hemopurifier has been shown to reduce the number of EVs in cancer patient plasma samples.
As a reminder, the primary endpoint for the approximate 9 to 18-patient study is safety. The trials will monitor any adverse events and clinically significant changes in lab tests of Hemopurifier treated patients with solid tumors with stable or progressive disease at different treatment intervals. Patients who do not respond to the PD-1 antibody therapy will be eligible to enter the Hemopurifier period of the study where sequential cohorts will receive 1, 2, or 3 Hemopurifier treatments during a one-week period.
In addition to safety, the study includes exploratory analyses evaluating how many Hemopurifier® treatments are needed to decrease the concentration of EVs, and if these changes in EV concentrations improve the body’s own natural ability to attack tumor cells. These findings are intended to guide the design of future safety and efficacy trials, including a potential Premarket Approval (PMA) study required by the FDA and other global regulatory agencies.
Regulatory Approval India
On June 19, 2025, the Company received formal approval from India’s Central Drugs Standard Control Organization (CDSCO) to initiate a similar trial at Medanta Medicity Hospital. The approval followed a meeting with the Subject Expert Committee and prior Ethics Committee clearance. The trial will begin following a Site Initiation Visit (SIV) conducted by Aethlon’s India-based CRO, Qualtran.
Preclinical Study Supports Broader Applications
On May 12, 2025, the results from Aethlon’s preclinical ex vivo study were published in bioRxiv, and the manuscript has been submitted to a peer-reviewed journal for publication. Those results showed that the Hemopurifier, using proprietary Galanthus nivalis agglutin (GNA) affinity resin, removed 98.5% of platelet -derived extracellular vesicles (PD-EVs) from human plasma during a timepoint equivalent to a 4-hour HP treatment. Excessive levels of PD-EVs have been implicated in a myriad of diseases, including cancer, lupus, systemic sclerosis, multiple sclerosis, Alzheimer’s disease, sepsis, acute and Long COVID. The results of this study support the ongoing oncology trial in Australia and suggest potential applications of the Hemopurifier in other EV-associated diseases.
The manuscript describing this study has been submitted to a peer-reviewed journal for publication.
Scientific Collaboration in Long COVID Research
Aethlon’s collaborative research with the UCSF Long COVID Clinic was accepted for a poster presentation at the Keystone Symposium on Long COVID and Other Post-Acute Infection Syndromes (August 10-13, 2025). The study analyzed blood samples from participants with Long COVID as well as controls that had recovered from COVID-19 infection to evaluate the binding of larger and smaller extracellular vesicles to the Hemopurifier’s lectin affinity resin, respectively. These findings build on prior clinical evidence and support further investigation of the Hemopurifier in Long COVID, an unmet medical need affecting approximately 44 and 48 million people in the United States alone, with an estimated economic burden of 2 billion dollars in those with symptoms lasting a year.
Operational Achievements
In fiscal 2025, Aethlon streamlined operations and significantly reduced its operating expenses, positioning the company for sustained focus on its clinical and regulatory goals.
Financial Results for the Fiscal Fourth Quarter Ended March 31, 2025
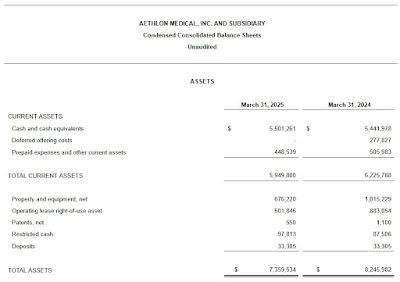
As of March 31, 2025, Aethlon had a cash balance of approximately $5.5 million.
Consolidated operating expenses for the fiscal year ended March 31, 2025, were approximately $9.3 million, representing a decrease of $3.3 million or approximately 26%, compared to $12.6 million for the fiscal year ended March 31, 2024. This reduction was primarily driven by lower payroll and related expenses, professional fees, and general and administrative costs.
Payroll and related expenses declined by an approximate $1.3 million, reflecting an approximate $900,000 reduction in salaries and related expenses and an approximate $800,000 decrease in stock-based compensation. These reductions were primarily attributable to the termination of three executives—one in the fiscal year 2024, one in July and October 2024—and a workforce reduction of non-executive staff in August 2024. The decrease in stock-based compensation was primarily due to the absence of accelerated vesting charges recognized in the prior year related to the termination of our former Chief Executive Officer, as well as reduced expenses following the departure of executives and staff. These decreases were partially offset by an increase of approximately $400,000 in severance expenses associated with the termination of two former executives.
Professional fees also declined by approximately $1.3 million. This decrease includes $600,000 in legal costs savings resulting from a transition to a new legal firm, and an approximate $500,000 related to the termination of services with a contract manufacturing organization and the completion of a project that involved using an outside lab to process samples. Consulting fees related to scientific projects and regulatory projects declined by approximately $300,000. These reductions were partially offset by an approximate $85,000 increase in accounting fees associated with obtaining audit firm consents for various securities filings.
General and administrative expenses decreased by approximately $660,000. The reduction was driven primarily by a $534,000 reduction in costs related to fewer raw material purchases, no cleanroom certification expenses, and reduced reliance on outside services for maintenance of the manufacturing facility. Laboratory supplies and testing costs also declined by $337,000 following the completion of oncology and transplant-related projects. Insurance expenses decreased by $141,000, reflecting lower medical and workers’ compensation premiums due to reduced headcount, as well as an overall decrease in business insurance costs. Additional reductions included $44,000 in travel and entertainment expenses, $24,000 decrease in office supplies, and $19,000 in depreciation expense related to the disposal of certain equipment. These decreases were partially offset by a $467,000 increase in clinical trial expenses associated with our ongoing oncology study in Australia.
As a result of the above factors, our operating loss decreased to $9.3 million for the fiscal year ended March 31, 2025, from $12.6 million for the fiscal year ended March 31, 2024.
Other Income (Expense)
Other expenses for the year ended March 31, 2025, included a non-cash charge of approximately $4.6 million related to a warrant inducement offer. In March 2025, we offered certain warrant holders the opportunity to exercise existing warrants at a temporarily reduced exercise price in exchange for the issuance of new warrants. The inducement expense recognized represents the combined fair value of the new warrants issued and the incremental fair value resulting from the modification of the exercise price of the existing warrants. This transaction did not impact cash flows from operating activities.
During the fiscal year ended March 31, 2025, we recognized approximately $324,450 in other income related to the Employee Retention Tax Credit (ERTC) under the CARES Act and subsequent legislation. We recorded the ERTC as other income in the periods in which the payments were received. In addition, we recognized $36,339 in interest income related to the ERTC during fiscal 2025. As of March 31, 2025, the remaining expected credit was recorded as a receivable within other current assets on our consolidated balance sheet. No amounts were recorded in the prior fiscal year.
The consolidated balance sheets for March 31, 2025 and March 31, 2024, and the consolidated statements of operations for the fiscal years ended March 31, 2025 and 2024 follow at the end of this release.
Management will host a conference call today, Thursday, June 26, 2025, at 4:30 p.m. ET to review the company’s financial results and recent corporate developments. Following management’s formal remarks, there will be a question and answer session.
Interested parties can register for the conference call by navigating to https://dpregister.com/sreg/10200578/ff60012c0c. Please note that registered participants will receive their dial-in number upon registration.
Interested parties without internet access or unable to pre-register may dial in by calling:
PARTICIPANT DIAL IN (TOLL-FREE): 1-844-836-8741
PARTICIPANT INTERNATIONAL DIAL IN: 1-412-317-5442
All callers should ask for the Aethlon Medical, Inc. conference call.
A replay of the call will be available approximately one hour after the end of the call through July 26, 2025. The replay can be accessed via Aethlon Medical’s website or by dialing 1-877-344-7529 (domestic) or 1-412-317-0088 (international) or Canada toll-free at 1-855-669-9658. The replay conference ID number is 4903201.
About Aethlon and the Hemopurifier®
Aethlon Medical is a medical therapeutic company focused on developing the Hemopurifier, a clinical-stage immunotherapeutic device which is designed to combat cancer and life-threatening viral infections and for use in organ transplantation. In human studies, the Hemopurifier has demonstrated the removal of life-threatening viruses and in pre-clinical studies, the Hemopurifier has demonstrated the removal of harmful exosomes from biological fluids, utilizing its proprietary lectin-based technology. This action has potential applications in cancer, where exosomes may promote immune suppression and metastasis, and in life-threatening infectious diseases. The Hemopurifier is a U.S. Food and Drug Administration (FDA) designated Breakthrough Device indicated for the treatment of individuals with advanced or metastatic cancer who are either unresponsive to or intolerant of standard of care therapy, and with cancer types in which exosomes have been shown to participate in the development or severity of the disease. The Hemopurifier also holds an FDA Breakthrough Device designation and an open Investigational Device Exemption (IDE) application related to the treatment of life-threatening viruses that are not addressed with approved therapies.
Additional information can be found at www.AethlonMedical.com.
Forward-Looking Statements
This press release contains forward-looking statements within the meaning of Section 27A of the Securities Act of 1933 and Section 21E of the Securities Exchange Act of 1934 that involve risks and uncertainties. Statements containing words such as "may," "believe," "anticipate," "expect," "intend," "plan," "project," "will," "projections," "estimate," "potentially" or similar expressions constitute forward-looking statements. Such forward-looking statements are subject to significant risks and uncertainties and actual results may differ materially from the results anticipated in the forward-looking statements. These forward-looking statements are based upon Aethlon's current expectations and involve assumptions that may never materialize or may prove to be incorrect. Factors that may contribute to such differences include, without limitation, the Company's ability to raise additional capital on terms favorable to the Company, or at all; the Company’s ability to successfully complete development of the Hemopurifier; the Company’s ability to successfully demonstrate the utility and safety of the Hemopurifier in cancer and infectious diseases and in the transplant setting; the Company’s ability to achieve and realize the anticipated benefits from potential milestones; the Company’s ability to obtain approval from the Ethics Committee of its third location in Australia, including on the timeline expected by the Company; the Company’s ability to enroll additional patients in its oncology clinical trials in Australia and India, including on the timeline expected by the Company; the Company’s ability to manage and successfully complete its clinical trials; the Company’s ability to successfully manufacture the Hemopurifier in sufficient quantities for its clinical trials; unforeseen changes in regulatory requirements; the Company’s collaborative research with UCSF Long Covid Clinic; the Company’s ability to further research potential applications of the Hemopurifier in other EV-associated diseases and other potential risks. The foregoing list of risks and uncertainties is illustrative but is not exhaustive. Additional factors that could cause results to differ materially from those anticipated in forward-looking statements can be found under the caption "Risk Factors" in the Company's Annual Report on Form 10-K for the year ended March 31, 2025, and in the Company's other filings with the Securities and Exchange Commission, including its quarterly Reports on Form 10-Q. All forward-looking statements contained in this press release speak only as of the date on which they were made. Except as may be required by law, the Company does not intend, nor does it undertake any duty, to update this information to reflect future events or circumstances.
Company Contact:
Jim Frakes
Chief Executive Officer and Chief Financial Officer
Aethlon Medical, Inc.
Jfrakes@aethlonmedical.com
Investor Contact:
Susan Noonan
S.A. Noonan Communications, LLC
susan@sanoonan.com
More info on AEMD at Investorideas.com Visit: https://www.investorideas.com/CO/AEMD/
Get News Alerts on Aethlon Medical
Disclaimer/Disclosure: Aethlon Medical, Inc. (AEMD) is a paid featured medical tech stock on Investor ideas More disclosure: Investorideas.com is a digital publisher of third party sourced news, articles and equity research as well as creates original content, including video, interviews and articles. Original content created by investorideas is protected by copyright laws other than syndication rights. Our site does not make recommendations for purchases or sale of stocks, services or products. Nothing on our sites should be construed as an offer or solicitation to buy or sell products or securities. All investing involves risk and possible losses. This site is currently compensated for news publication and distribution, social media and marketing, content creation and more. More disclosure: Contact management and IR of each company directly regarding specific questions.
Get more biotech and medical tech news, articles, podcasts and stock directories
About Investorideas.com https://www.investorideas.com/About/